In hospitalized people with COVID-19 pneumonia, a drug study indicated that therapy with the immunosuppressant interferon beta-1a plus with antibacterial remdesivir is not preferable to therapy using remdesivir individually.
Furthermore, researchers discovered found interferon beta-1a is linked to greater side effects and poorer results in a subset of individuals that needed high-flow oxygen. Such results are just reported in The Lancet Respiratory Medicine.
Adults With Covid Who Are Hospitalized Do Not Benefit From Interferon
To help patients recover from infection of Coronavirus, the experts have left no stone unturned. Hence, a number of therapies tried by them on different samples where few are proven almost of no use or ineffective.
Among such therapies, one is Interferon which is checked by a team of experts.
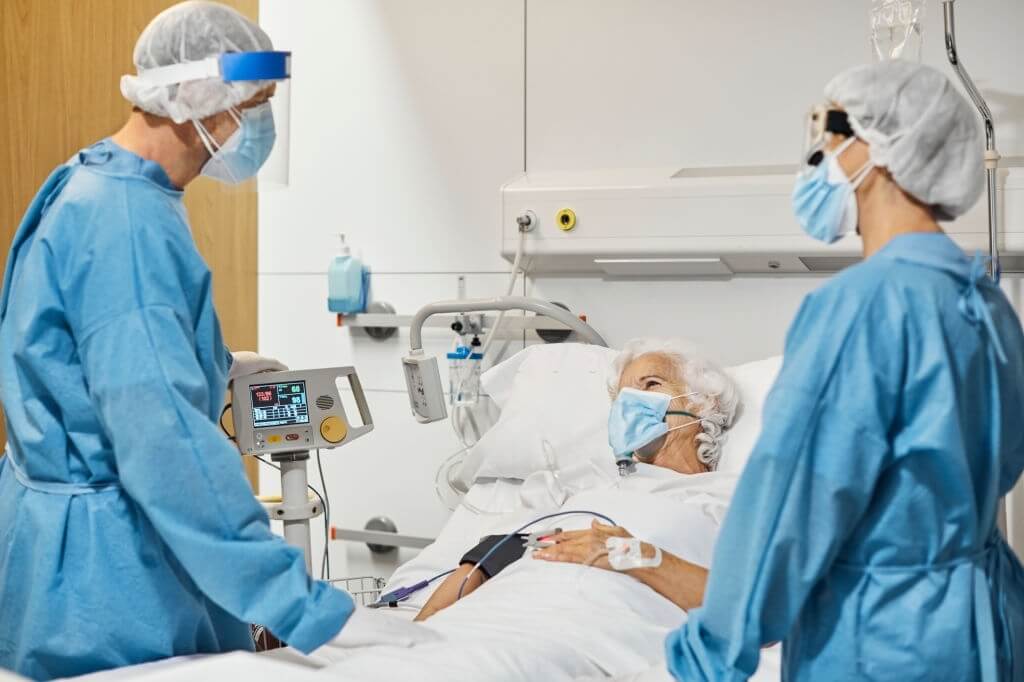
From August 5, 2020, through December 21, 2020, researchers conducted the Adaptive COVID-19 Treatments Trial 3 (ACTT-3). The National Institute of Allergy & Infectious Diseases (NIAID), which is a component of the National Institutes of Health, organized & supported the event.
Interferon-beta-1a shares the identical amino acid structure as interferon beta, a spontaneously occurring protein that belongs to the type 1 interferon family. Type 1 virions are produced by diseased tissues to aid the immunological systems in fighting infections, particularly viruses. Combination antiviral or anti-inflammatory effects are seen in genes associated.
The typical type 1 interferon reaction is inhibited following infection with SARS-CoV-2, the viruses that produce COVID-19, according to lab research. In contrast, prior investigations of COVID-19-infected hospitalized individuals found that many individuals produced less Interferon throughout reaction to SARS-CoV-2 infections, which is linked to higher serious illness. Therapy using interferon beta-1a may enhance medical results in persons with COVID-19, according to other lab research and medical evidence.
Nevertheless, in hospitalized individuals with COVID-19, the ACTT-3 researchers discovered the interferon beta-1a plus remdesivir is not linked with a medical advantage when contrasted to remdesivir solo. The key result, time to recuperation, remained the equivalent for all who received interferon beta-1a + remdesivir and individuals who received remdesivir alone (median five days). The chances of medical recovery at day 15 were likewise comparable between the two therapy subgroups.
Remdesivir is utilized as an effective treatment in this research since the antiretroviral is proven to be better than placebo in reducing the duration of recuperation in individuals hospitalized with COVID-19 in the initial iteration of ACTT studies.
EMD Serono Inc. manufactures and markets injectable interferon beta-1a for multiple sclerosis with the brand Rebif in the United States. Gilead Sciences, Inc., of Foster City, California, manufactures Remdesivir, also known as Veklury.
In summary, the results of this study suggest that IFN-1b is efficacious versus the new COVID-19 virus. IFN- has now been demonstrated to reduce total hospitalization time and the degree of COVID-19 respiratory complaints when combined with the existing course of treatment. In extreme situations, several trials have shown a reduction in ICU stay, improved survival rates, and lowered the requirement for invasive external breathing when contrasted to controls.
Neuropsychiatric symptoms, diarrhea, heat, vomiting, and modest ALT increases were all reported as side events in COVID-19 individuals who received IFN-. There is one incident of hypersensitivity.
The results of the research above demonstrate that introducing IFN- to the therapy of COVID-19 individuals in hospitals has a favorable effect. To accurately determine the survival effect of IFN-1b, more randomized medical studies with a high population number were required.
As a result, it was unable to accurately assess the result & impact of interferon-beta just on infection. An additional disadvantage is the absence of scholarly materials from previous COVID-19 research. In the future, larger population numbers but also longer thorough trials can aid in determining if individuals receiving IFN- therapy have a better mortality probability.