Scientists from the SWOG Cancer Researchers Network, a cancer drug development organization supported by the National Cancer Institute, part of the National Institutes of Health, carried out the study. The findings were reported in the Journal of Cancer Immunotherapy.
Four individuals’ tumors reacted partly or fully to medication with a combo of the immunology medications ipilimumab & nivolumab in a recent cohort of 16 sick people with both the uncommon disease angiosarcoma. Two more individuals were able to keep their illness constant while using the treatment mixture. In rare instances, therapeutic results had lasted more than a decade, and at that one person has had his tumors completely vanish.
Immunotherapy Reaction In Uncommon Angiosarcoma Tumours
The study was led by Michael Wagner, MD, of the Fred Hutchinson Cancer Research Center and the University of Washington. “Angiosarcoma is rare cancer that has few effective therapy options that benefit patients for a long time,” Wagner says. “If there’s a subset of patients and it seems like there are who will have a durable response with immunotherapy, these patients would hopefully live longer and have a good quality of life.”
The research is conducted as part of the groundbreaking DART experiment, a government-subsidized “basket” trial that examines the efficacy of combining ipilimumab with nivolumab vs a variety of uncommon malignancies. The NCI and Bristol-Meyers Squibb, the manufacturer of these treatments, have a Cooperative Development and Production Contract that provides such experimental medicines, also called immunotherapy.
The immunotherapy reaction has been noted on different patients by the experts and found the results of this treatment better on different patients however, in some cases the results vary as per the severity of the medical condition of patients. These medicines have proven highly effective due to the combination that affects the spread and control of this tumor.
This medical condition is not a regular one and not many of the options are present with the medical fraternity also. Hence this result of the combination of medicines can be a breakthrough in this field and prove useful to a number of patients who suffer from the same.
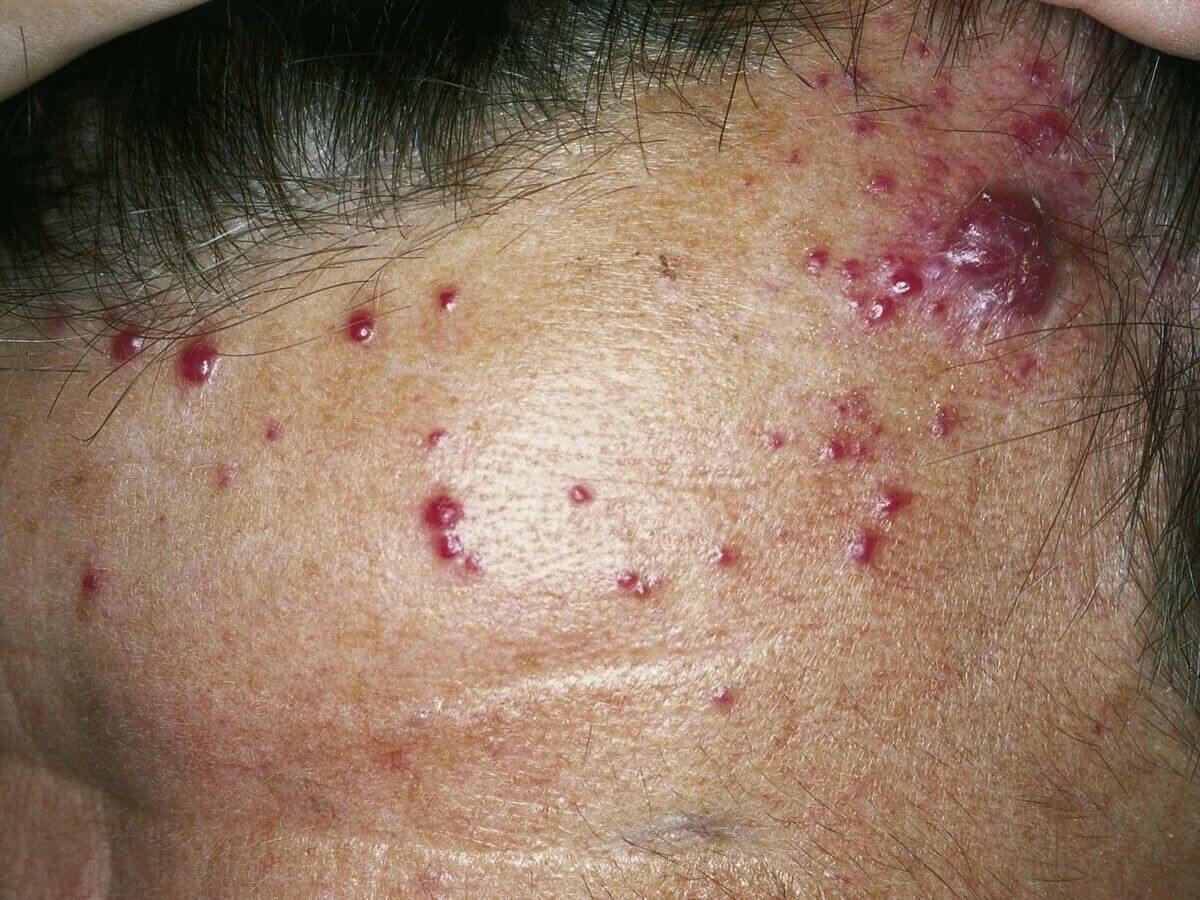
Angiosarcoma is an ultrarare tumor that primarily forms in the epidermis and is extremely malignant, having just around 400 additional instances recorded in the United States each year. Angiosarcoma is commonly managed with treatment after it has spread, although the tumor reaction to the medication is generally short, and angiosarcoma does have a high fatality risk.
Other clinical studies had reported isolated occurrences of angiosarcoma tumors reacting to therapy, but this is the only planned study of immunotherapeutic in the condition.”Outside of DART, the angiosarcoma study probably would have taken years to get going,” Wagner says. “Adding it to DART helped expedite getting these results.”
Immuno circuit medicines are known for causing adverse symptoms, and medication toxicity in this set of individuals is similar to that found in those other sarcoma studies using the ipilimumab & nivolumab combo. 75 percent of these individuals experienced therapy side effects, and 25percent reported stage iii or higher therapy incidents.
Wagner’s group used the immune combo to treat 16 individuals who were suitable. Six of the individuals had their tumors decrease slightly, 3 of them fulfilled the criterion for a complete reaction to therapy, but one achieved the criterion for a full reaction to the diagnosis.
Following beginning therapy, 2 more individuals’ tumors remained constant, and those 2 trials are still receiving therapy and have a stable condition.3 of the 5 people with basic dermatological illness of the forehead or scalp who took part in the study recovered after therapy. This is a group of people who have an especially poor prognosis given existing therapies.