It is a well-known fact that the protection offered by the two doses of mRNA vaccines by Pfizer and Moderna wanes over time. Considering this fact, the US FDA has already authorized the use of a booster shot of the vaccine for immunocompromised patients last week. However, considering the rapid spread in the delta variant of the virus throughout the country even amongst those vaccinated, officials are now evaluating the possibility of authorizing a booster dose for people already vaccinated.
According to data released by the leading vaccine manufacturers Pfizer and Moderna, a third dose elicited a significantly higher antibody response against the coronavirus compared to people with two doses.
Most Americans To Be Eligible For Covid Booster Shot
The third dose is believed to offer significant protection even against the delta and beta variants of the virus.
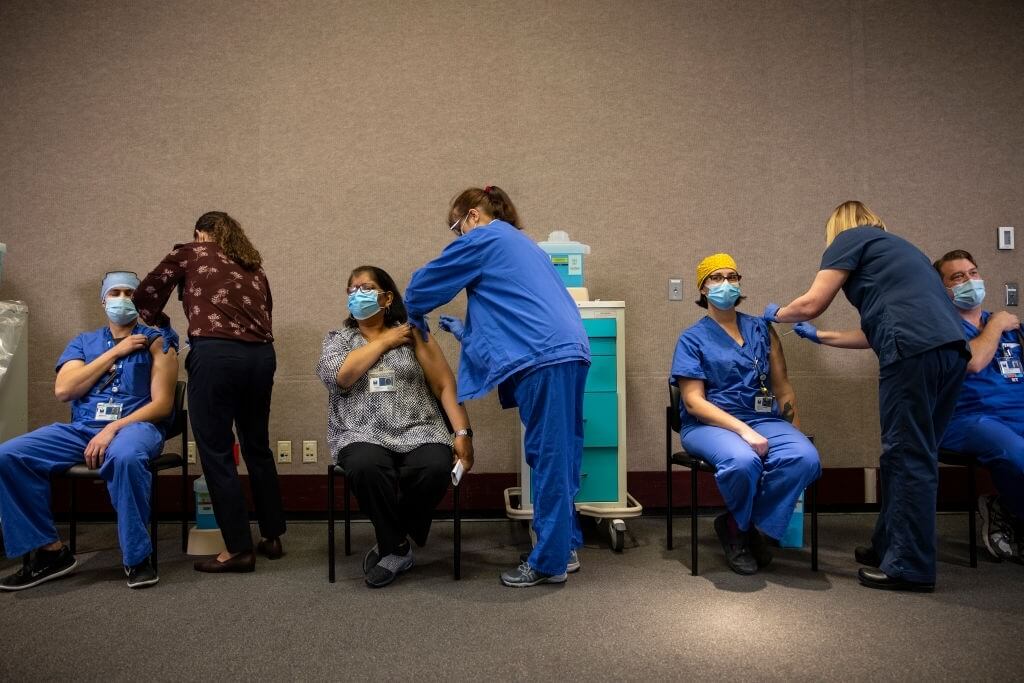
According to officials at Moderna and Pfizer, a booster dose given between 6 to 12 months after the primary vaccination schedule may help maintain a higher level of protection against Covid-19.
Based on information received from top healthcare officials in the Biden administration, a plan is being formulated to begin administering a third dose of vaccine to Americans eight months after becoming fully vaccinated. It is believed that the two mRNA vaccine manufacturers – Pfizer and Moderna have submitted initial data to the FDA supporting the use of Covid-19 Booster doses.
Countries like Israel have already started providing booster doses for their citizens and other developed nations are exploring the idea at this stage. It is believed that authorization for the booster dose could be granted as early as this week. Administration of the booster doses shall begin only once the US FDA approves of this.
Following the authorization of booster dose, the frontline healthcare workers, nurses, elder Americans who have been vaccinated in December 2020 shall become eligible for the booster shot in the beginning. Others who have been given the vaccination in early 2021 and the latter part of 2021 shall become eligible gradually. The 8 months’ time duration is still being evaluated and discussed.
At this stage, even though close to 168 million Americans are fully vaccinated the spread of the delta variant is extremely aggressive on account of both spread of the virus amongst the unvaccinated community and the “breakthrough infection” amongst those fully vaccinated. The US believes that it has enough domestic supply of vaccines to offer booster shots to the majority of Americans.
The current decision to roll out booster shots is only for the Pfizer and Moderna vaccine shot. Officials are still gathering data for the Johnson & Johnson vaccine and the decision on the J&J booster vaccine shall take a few more weeks for evaluation.
In response to this news, the global healthcare experts and the World Health Organization officials have called upon the developed and the wealthier nations to hold off booster shots for their citizens. They want the globally short Covid-19 vaccine shots to be directed to the developing nations where citizens are yet to receive their first doses.