The VISION Networks of the CDC collected information from greater than 201,000 admissions in 10 states. Within this subgroup, around 7,000 persons met the report’s requirements.
The researchers looked at how many unvaccinated people had high COVID-19 testing greater than three months earlier getting brought to the clinic for the viruses, as well as how many people got the Pfizer and Moderna vaccination but were never identified as COVID before getting treated.
COVID Immunizations Reduce COVID-Related Hospitalization
In different researches, the experts have found that immunity is the best way to keep one protected against the infection of this virus. Hence, various options are being tested by them to improve the immunity of people so that the infection and spread of the virus can be controlled in a short span.
The vaccines offered are also nothing but an option to improve the inner strength of the body that can counter the infection spread by the virus.
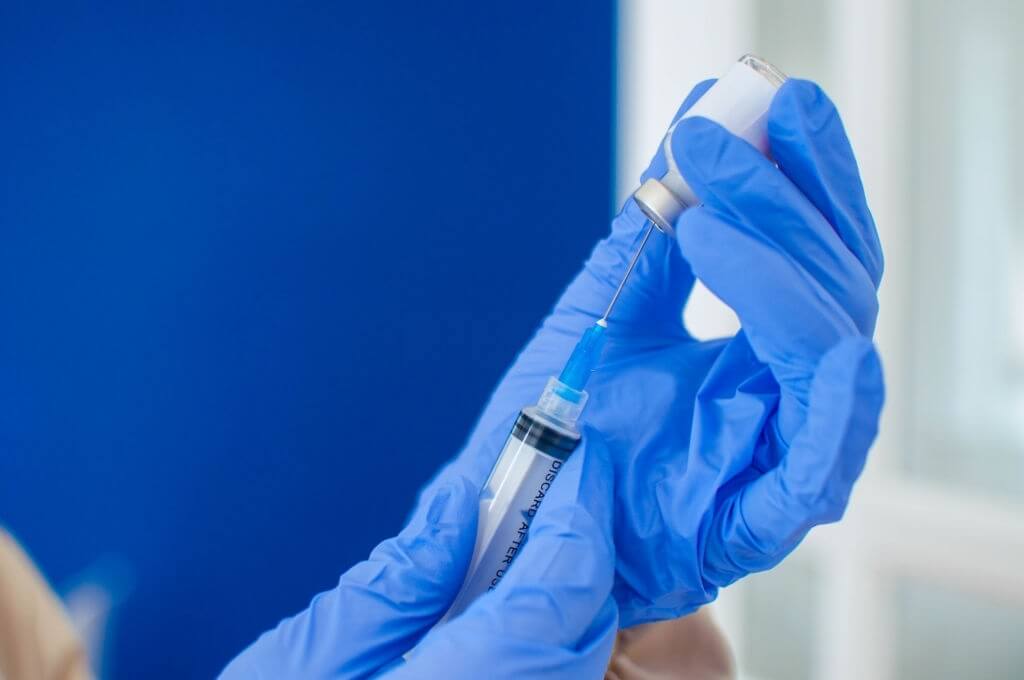
Non-vaccinated individuals having a prior COVID-19 illness are five times greater probable than immunized people to be hospitalized, according to the study.
According to nationwide research conducted by the Centers for Disease Control & Prevention (CDC), mRNA COVID-19 vaccinations are linked to much higher resistance over previous COVID-19 infections.
“This data provides powerful evidence that vaccinations offer superior protection against COVID-19 than relying on natural immunity alone,” said Shaun Grannis, M.D., M.S., vice president for data and analytics at Regenstrief Institute and professor of family medicine at School of Medicine.
“Many have been asking if they should get vaccinated if they’ve already been infected—this research shows the answer is yes.” Regenstrief contributes data and expertise to the VISION Network.
Altogether mRNA vaccinations are roughly 20 times better efficiency than previous infections simply in avoiding admissions amongst persons above the age of 65, according to the statistical study.
Lab research suggests that mRNA vaccinations produce significant amounts of antibodies, but individuals who recovered with COVID-19 have varied amounts of a monoclonal antibody, particularly if they had minor signs or are asymptomatic.
The CDC’s Morbidity and Mortality Weekly Report published a report entitled “Laboratory-Confirmed COVID-19 Among Adults Hospitalized with COVID-19–Like Illness with infection-induced or mRNA Vaccine-Induced SARS-CoV-2 Immunity—Nine States, January–September 2021.”
COVID-19 would remain to be a hazard to global public security till the majority of the world’s populace is immunized against it, and possibly treatment-resistant variations may evolve.
The treatment and treatment of this extremely contagious pulmonary viral infection necessitates a multidisciplinary and interprofessional strategy involving doctors of various specialties, pharmacists, chemists, public health professionals, and state officials.
When treating individuals having COVID-19, healthcare practitioners, pharmacists, and care professionals must maintain a closed-loop communications system.
Diagnostic suppliers on the frontlines trying to manage COVID-19 sick people must stay current on the newest medical guidance for diagnoses and restorative choices obtainable in the planning of COVID-19, particularly in light of the resurgence of fresh SARS-CoV-2 variations that can have a significant effect on incidence and death.
Individuals who appear with extra-pulmonary signs in the lack of lung signs should have a strong index of concern if they are in a highly exposed region or have recently been to a high-risk location. SARS-CoV-2 testing & prioritization must be performed on such individuals.
To stop the transmission of this disease, more tools for connection tracking and screening are needed.
According to CDC recommendations and provincial and local agencies’ COVID-19 procedures, sufferers should be informed and urged to follow social distance, travel restrictions, and the usage of masks.
Pharmacists should therefore stay informed regarding new medications that have been licensed or given urgent usage permission to treat COVID-19.