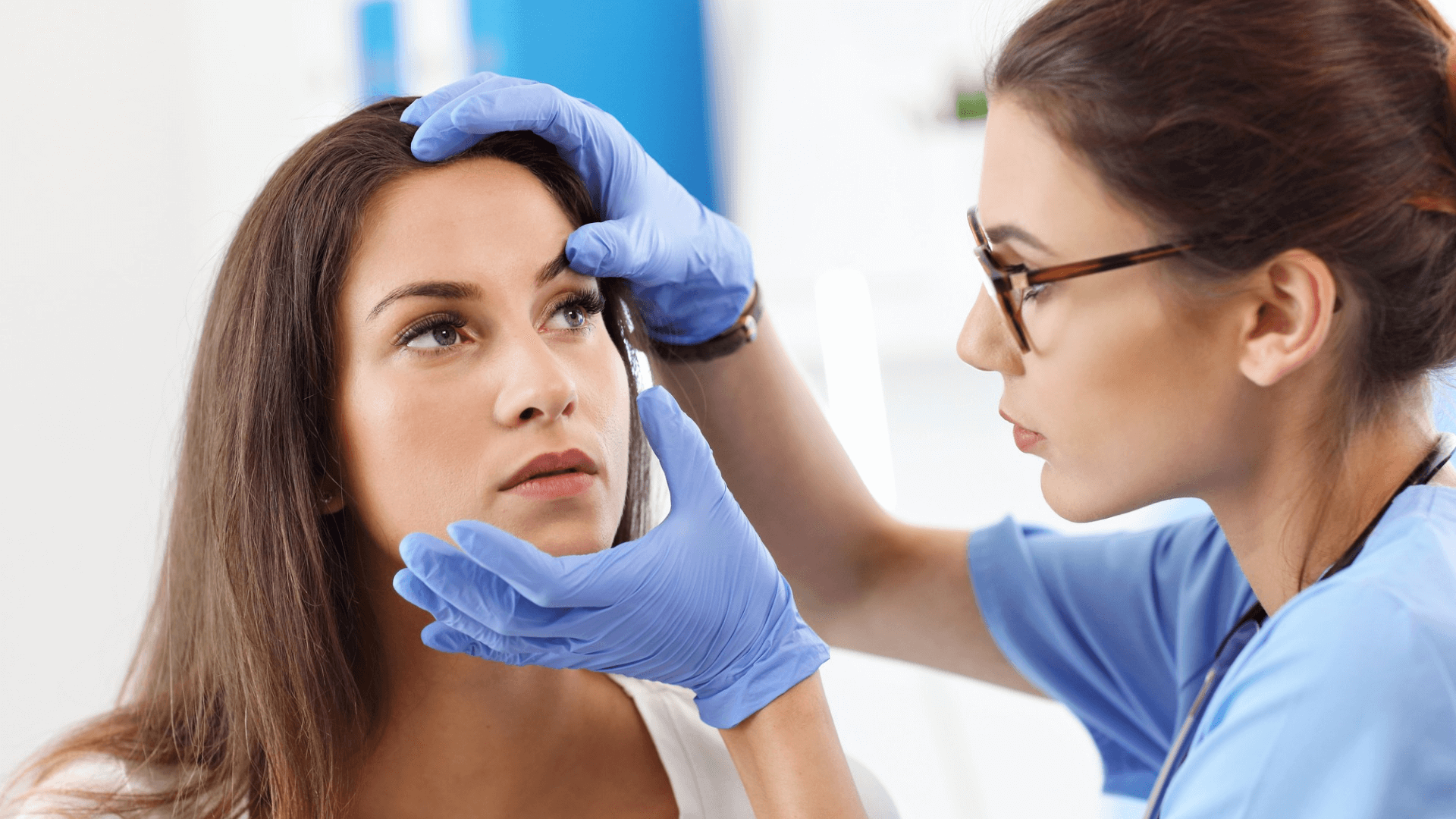
Tepezza (teprotumumab) was approved by the US Food and Drug Administration last year to treat thyroid eye disease a rare condition in which the muscles and fatty tissues behind the eye become inflamed causing the eyes to be pushed forward and bulge outwards. This new treatment is especially exciting because it is the first and only prescription drug for the treatment of thyroid eye disease potentially saving some people from surgery.
Even patients with mild inflammation can experience a reduction in eye bulging according to a new study presented on November 15 at AAO 2021 the 125th annual meeting of the American Academy of Ophthalmology. The symptoms of the disease are caused by inflammation which begins the disease. The inflammation decreases or clears as the disease progresses but the pain eye bulging and double vision may persist.
A Groundbreaking Treatment For Mild Thyroid Eye Disease
Thyroid eye disease is an immune system disorder that affects approximately 19 out of every 100000 people each year. Eye irritation pain and pressure dry or irritated eyes, and light sensitivity are just some of the symptoms that can be caused by a condition known as blepharitis. Symptoms can range from mild to severe. It can also cause vision loss though this is uncommon.
Even though the percentage of people affected with this condition is very small, it cannot be neglected as it can lead to severe complications in some patients. In this regard, researchers are working hard to avoid surgery as much as possible. The new treatment sounds exciting at this stage and further developments are expected in the next few months according to experts. It will bring a new ray of hope for many patients.
Researchers wanted to see if Tepezza could reduce eye bulging in patients with lower levels of inflammation than those evaluated in the two pivotal randomized trials that led to Tepezza’s approval by the FDA.
“We know from the Phase 2 and 3 clinical trials that Tepezza is effective in reducing proptosis in thyroid eye disease patients with high inflammatory activity and are pleased to see comparable efficacy in those with low-level inflammation,” said Raymond Douglas MD Ph.D. study investigator and director of the Orbital and Thyroid Eye Disease Program. “This analysis is encouraging for physicians looking to prescribe Tepezza for patients who have little to no inflammatory activity but are still living with other devastating symptoms that affect their everyday lives.”
They looked at 15 patients with a clinical activity score of 4 or lower. More than 86 percent of those who received treatment for six months saw their proptosis (eye bulging) go down by 2.9 millimeters on average. Patients with a clinical activity score of less than 3 responded to treatment 83.3 percent of the time with a mean reduction in proptosis of 2.6 1.4 mm.
TED is a complex autoimmune disease that can cause vision loss disfigurement and a decrease in quality of life. Advances in our understanding of TED pathophysiology have resulted in a paradigm shift in TED management. Historically the mainstays of treatment were corticosteroids radiation therapy and surgical correction.
Teprotumumab was approved by the FDA for the treatment of TED in 2020 after pivotal phase 2 and 3 RCTs revealed significant improvements in proptosis diplopia QoL and CAS. Teprotumumab is a promising treatment for patients with active moderate to severe TED especially those who have proptosis and/or diplopia. Studies of long-term efficacy are still needed to better understand the long-term effects of all treatment options.
Researchers have recently shifted their focus to developing additional targeted molecular therapies. For the time being it’s not clear how TED’s treatment paradigm will evolve in light of new therapeutic options.