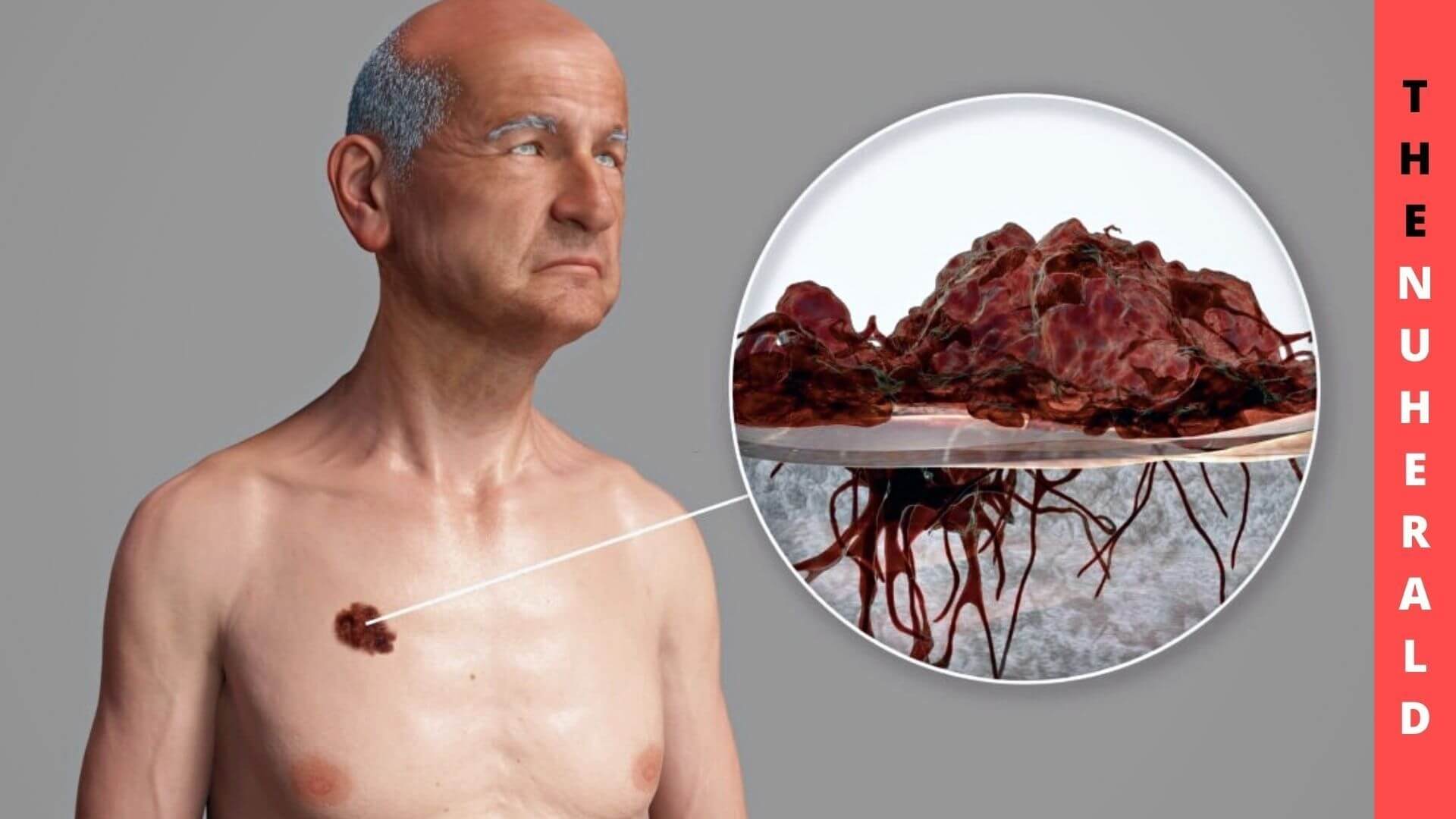
Alterations drive tumor genesis and treatment resistance in DNA within and beyond chromosomes. Despite the attribute that precision therapies have improved the survival rates for melanoma patients and others with cancer, the tumors often don’t respond to those treatments.
Study Claims To Reveal What Makes Melanomas Resistant To Drugs
UCLA Jonsson Comprehensive Cancer Center developed a melanoma drug resistance model to study alterations in a pathway that promotes cancer. In addition to allowing experimenters to explore intrachromosomal changes involved in opposition in cancer partitions, this model also permitted experimenters to study configurations and dynamics associated with these changes.
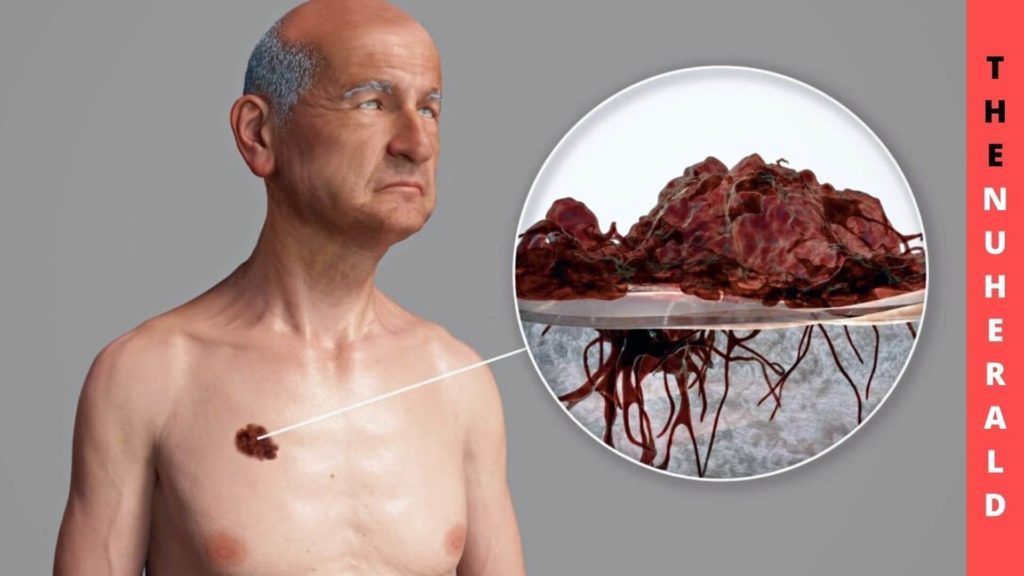
They could also impact drug dosage regimens and follow response-related reactions with the model, which may lead to a therapeutic approach that will be more comprehensive efficacy.
It is well comprehended that cancer genomes, particularly those of aggressive cancers, can undergo rapid and unexpected changes. Our understanding of conditions, arrangements, Interactions between genes and chromosomes that affect cancer development and resistance to drugs is rapidly developing due to numerous advancements in genetic analysis, map-making, and analytics technologies.
An article published online on Dec. 20 in Cancer Discovery describes Focused amplification mechanisms cancer-promoting genes BRAF and MAPK in the MAPK pathway, which is involved in different types of cancer, according to Thomas Graeber, Ph.D., senior author.
Several drugs, including vemurafenib and dabrafenib, inhibit the MAPK pathway in cancers with BRAF mutations, including melanoma and bowel cancer. Resistance, nevertheless, often results from restoring this course or activating another path, and Melanoma often acquires BRAF amplification due to reactivation of the MAPK pathway.
Inhibiting targeted therapies can occur when amplicons, portions of chromosomal DNA that undergo amplification, are overexpressed. Although DNA cannot be detached from the chromosomes in a normal human cell, it can be found in the center. Due to how these amplicons occur under the microscope, they have initially been called double minutes (DMs).
As time progresses, some regions can be reintegrated, becoming homogeneously stained areas (HSRs) since they possess rows of amplicons aligned vertically. Understanding this research, there will be new possibilities in the medical field after all. So, considering each factor is very much important.
The switch over to DM and HSR modes in focal amplification has been celebrated for more than 60 years. Still, technology has only recently revealed its proper form, institution, and part in tumor growth. Nonetheless, both forms of focal amplification are known to contribute to cancer development and drug resistance.
Kai Song, a graduate scholar and first author of the article, explained that the purpose of the study was to provide details about the layout, dynamics, and vulnerability of focal amplifications of MAPK in Melanoma. To develop restorative practices that overpower resistance, we need to understand these arrangements and interactions in-depth to apprehend tumor evolution and plasticity.
In their study, the team targeted the MAPK signaling pathway with kinase inhibitors to establishing a model of drug resistance. During months of culture at a stable dose, amplification of the BRAF gene originally appeared in extrachromosomal DNA but eventually evolved into intrachromosomal HSR. They note that Melanoma is a cancer that switches modes consistent with other studies that report similar results in leukemia and neuroblastoma with focally amplified oncogenes.
Researchers found that tumor cells maintained particular advantages due to the different types of DNA, such as those related treatment conditions that can be changed. Under steady, controlled drug-dose essentials, intrachromosomal HSRs seem to be more effective at promoting cancer growth than extrachromosomal HSRs, which is evidence that fitness-driven evolution can transfer DMs to HSRs.
Regardless, an oscillating or non-steady drug dose challenge may be beneficial as the amplicon composition number isn’t connected to chromosomes, which results in the retention of DM-containing cells.